Abstract
Introduction
Myristoylation is the post-translational modification of proteins with a 14-carbon fatty-acid by N-myristoyltransferase 1 (NMT1) or NMT2 [1]. Myristoylation is key to protein membrane binding and activity, and cell survival. Bioinformatics data suggests NMT mRNA expression relates to overall survival (OS) in AML [2]. We characterized NMT protein levels in AML patients.
Methods
We performed an exploratory retrospective and prospective cohort study of adult patients with newly diagnosed AML (excluding acute promyelocytic leukemia) at the University of Alberta Hospital from 2014-2016, and a group of non-leukemic controls. We assayed marrow aspirates for NMT1/2 protein levels by fluorescence activated cell sorting with intracytoplasmic staining by custom mouse anti-NMT1-Alexa-Fluor-647 or anti-NMT2-FITC, expressed as Mean Fluorescent Intensity (MFI) relative to IgG1k isotype control. NMT1/2 MFI was determined in bulk, CD34+, and CD34+38- blast subpopulations, if sufficient events permitted. Clinical data was analysed by t-test or ANOVA, with a Bonferroni correction for multiple comparisons. Relapse-free survival (RFS) and OS were dichotomized by a receiver operator curve, followed by Kaplan-Meier analysis with a log-rank test for significance or univariate and multivariate Cox regression, p < 0.05 as significant. Censoring, survival, and clinical parameters were defined by European LeukemiaNet (ELN) guidelines and REMARK criteria. [3-4]
Results
A total of 104 AML patients were evaluated, 58 of whom received induction chemotherapy, in addition to 16 controls. Median age was 67.1 years, with 57 ELN intermediate-risk patients. Median OS and follow-up were 0.95 years and 1.71 years, respectively. NMT1 MFI was consistent and similar across all samples. NMT2 MFI was higher in mature lymphocytes vs. mature monocytes (mean MFI 3.01 ± 0.08 vs. 0.98 ± 0.03, p < 0.001). NMT2 MFI was higher in control CD34+ cells vs. CD34+ AML blasts (mean MFI 1.65 ± 0.11 vs. 1.21 ± 0.03, p < 0.001). NMT2 MFI in AML CD34+ blast subsets was higher with inv(16) (mean MFI 1.67 ± 0.05, p < 0.001) and lower with mutated NPM1 (mean MFI 1.09 ± 0.04, p = 0.004). NMT2 MFI was not associated with ELN risk group (p = 0.166) or achievement of complete remission (p = 0.303).
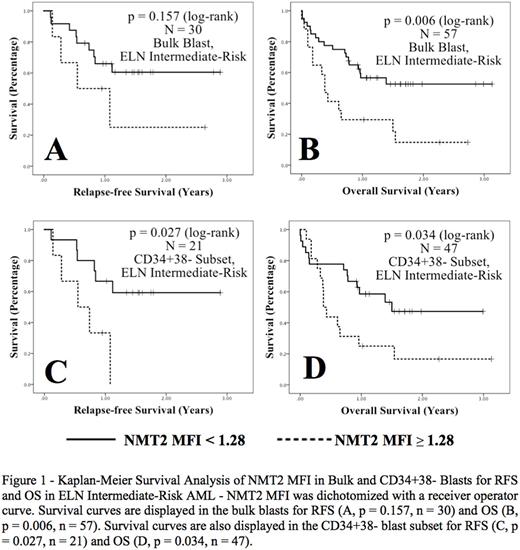
An NMT2 MFI receiver operator curve for OS in ELN intermediate-risk AML in CD34+38- blast subsets gave a cut-off of 1.28 (AUC 0.697, p = 0.021), with sensitivity 50% and specificity 80%. Using this cut-off in ELN intermediate-risk patients, a higher NMT2 MFI was significantly associated with inferior OS in the bulk blasts (p = 0.006) and inferior RFS (p = 0.027) and OS (p = 0.034) in the CD34+38- blast subsets (Figure 1). In ELN intermediate-risk AML, using age and WBC as covariates, higher NMT2 expression in bulk blasts was significantly associated with an inferior RFS on multivariate analysis, (HR 4.753, 95% CI 1.155 - 19.561, p = 0.031).
Conclusions
NMT2 protein expression by flow cytometry was significantly lower in CD34+ AML blasts compared to normal CD34+ cells, and was higher in inv(16) and lower in NPM1 mutated samples. Higher NMT2 expression predicted inferior RFS and OS in ELN intermediate-risk patients, with this effect being driven by the CD34+38- blast subsets. This may represent an indirect measure of the stemness of the blast population. NMT2 MFI is a novel, protein based, highly translatable biomarker for prognostication in intermediate-risk AML, and further investigation is warranted. The differential expression in CD34+ AML blasts makes this a potential therapeutic target.
References
[1] Martin, DDO, Beauchamp, E, Berthiaume, LG Biochimie 201193;18-31
[2] Vincent, K, Postovit, L, unpublished data 2016
[3] Dohner, H, Estey, EH, Amadori, S et al Blood 2010 115;453-474
[4] McShane, LM, Altman, DG, Sauerbrei, W et al Br. J. Cancer 2005 93;387-391
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal